About Us

Medclin Research Private Limited is a leading Clinical Research Organization (CRO) specializing in clinical trials, medical research, and evidence-based healthcare solutions. We are driven by a team of skilled medical professionals and experts with vast experience in clinical research and allied fields. Our core focus is on delivering transparent high-quality, data-driven clinical insights that support both medico-marketing strategies and clinical practice.
Beyond clinical trials, Medclin Research excels in epidemiology studies and public health surveys. We aim to improve public health by providing actionable insights that contribute to informed decision-making in healthcare. Our studies support a deeper understanding of disease prevalence, risk factors, and healthcare outcomes, which help shape evidence-based decision making for pharma companies.
Our Legacy
Medclin Research was established with the vision of creating a meaningful connection between scientific research, clinical practice, and healthcare strategy. With a strong foundation in clinical research and prescription-based insights, the organization has evolved into a focused clinical research partner supporting pharmaceutical and healthcare companies through high-quality clinical trials and real-world evidence (RWE) studies.
At Medclin, we believe that credible research begins with transparency, ethical conduct, and scientific rigor. Our approach emphasizes collaborative protocol design, close engagement with medical professionals and key opinion leaders, and the generation of reliable clinical data that reflects real-world practice. This integrated methodology enables pharmaceutical organizations to gain deeper insights into treatment patterns, clinical outcomes, and patient needs.
Over the years, Medclin Research has developed expertise across multiple therapeutic areas, supporting evidence generation that helps strengthen clinical confidence, guide strategic decision-making, and ultimately contribute to improved healthcare delivery. Our commitment remains focused on producing research that is not only scientifically robust but also relevant to real clinical settings and patient outcomes.
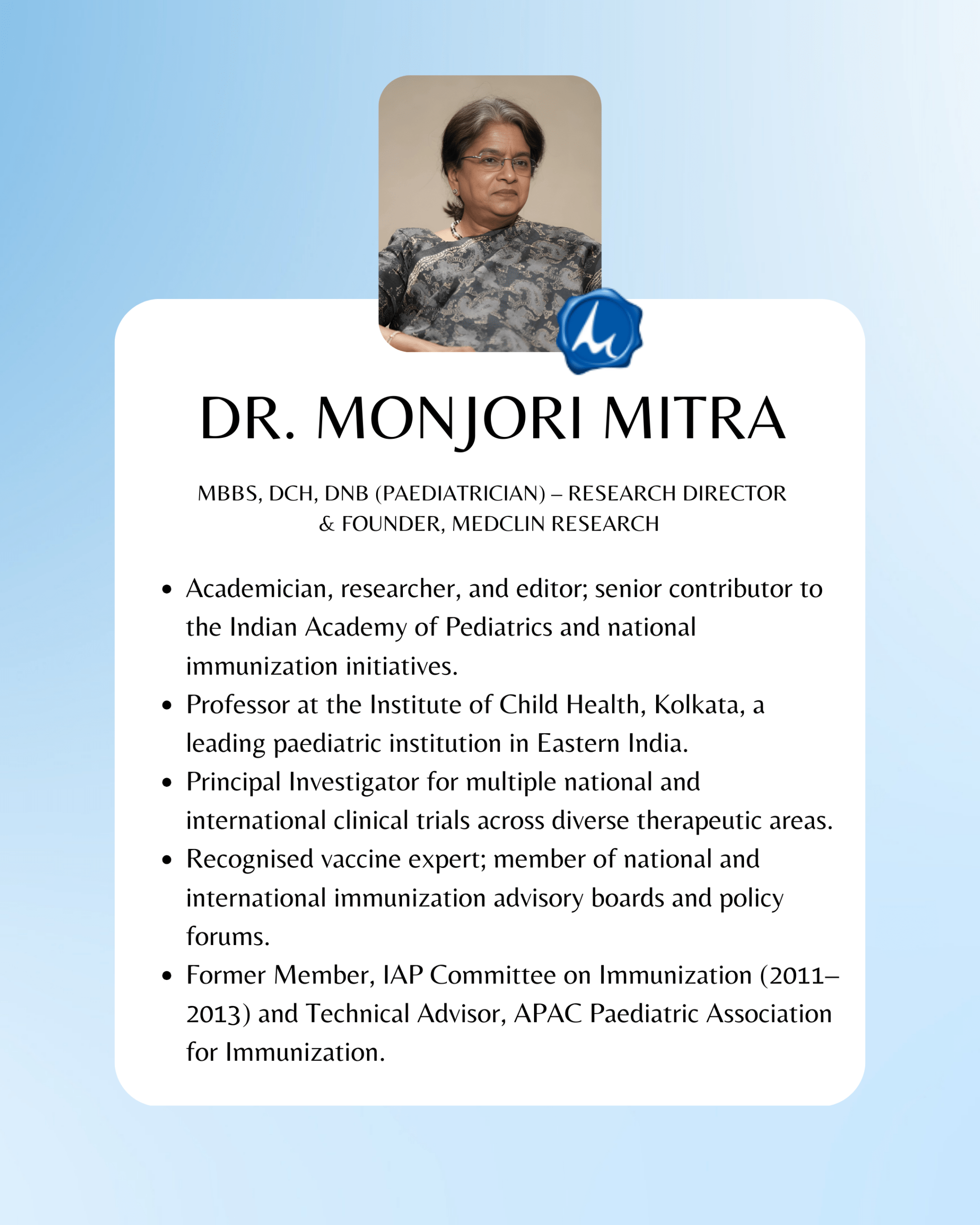
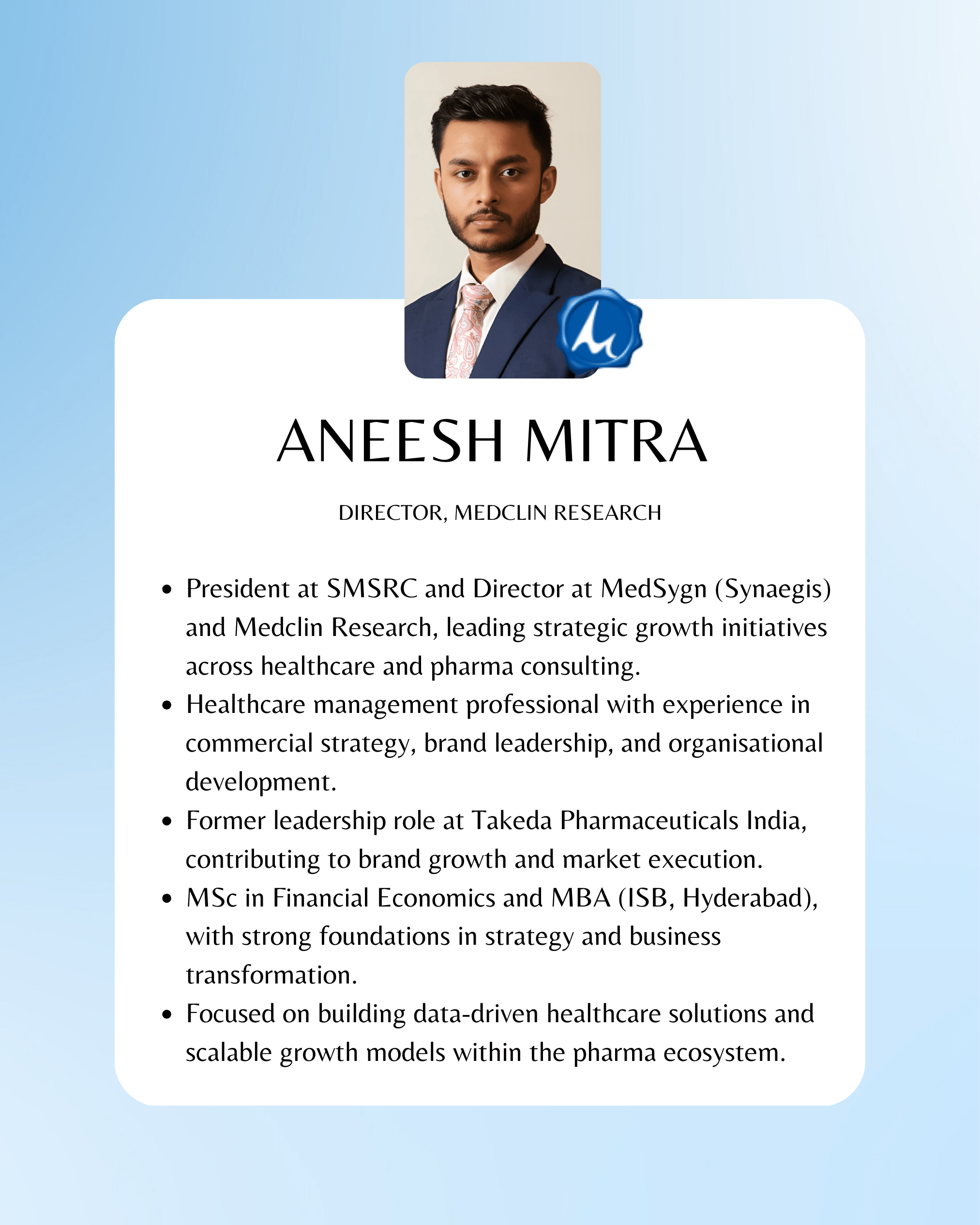
Founders
35 Years of Legacy
Book Your Appointment With Our Team
Connect with the most trusted CRO team
Our Impact in Numbers
Trusted by Pharma Giants














Clinical Research Partner of Choice
What we are Known for
- ✅ ISO 9001:2015 certified & GCP compliant
- ✅ Advanced technology: Inhouse EDC, MedSygn EMR
- ✅ Access to 10,000+ verified investigators across specialties
- ✅ Proven expertise in Phase II–IV trials and RWE studies
-
Track record of 80+ peer-reviewed publications Strong medical leadership
Phase II–IV trials conducted across multi-site networks with full regulatory support, monitoring, data management, and medical oversight.
Design and execution of cross-sectional and longitudinal studies using prescription data, EMRs, and physician networks.
Development of medico-marketing content, KOL engagement, consensus papers, and scientific publications.
Support for investigator-driven research through study design, data collection, and regulatory compliance.